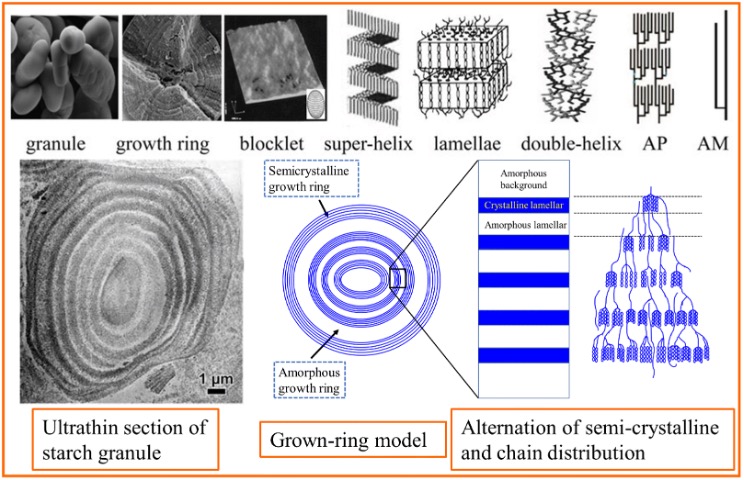
Starch Structure
Starch refers to the granules that store glucose polymers in plants. These granules are based on a semi-crystalline structure formed by the spatial arrangement of amylopectin polymer, which extends from the center of the granule to its surface. Amylose molecules are also distributed within this structure. Crystalline regions are formed due to the double-helix formations of amylopectin branches. These crystalline structures have nanometer-scale dimensions, and in these crystalline granules creates alternating crystalline and amorphous layers within the granule. As a result, starch does not dissolve in cold water and absorbs water only in minimal amounts. However, this characteristic leads to specific starch properties, such as swelling in hot water and the formation of high viscosity.
Temperature-Dependent Changes
The crystalline structure of starch is dependent on temperature and humidity. When sufficient moisture is present, and the temperature exceeds the gelatinization point, water molecules infiltrate the crystalline regions and double-helical structures. The crystals “melt,” and molecular mobility increases. This process is the beginning of starch gelatinization and the formation of a paste. It is an endothermic process due to the change in molecular energy. Equipment such as Differential Scanning Calorimetry (DSC) can detect these energy changes.
Differential Scanning Calorimetry (DSC)
DSC is a technique used to measure the electrical heat energy required to induce temperature changes in a sample compared to a reference sample. The sample, weighing only a few milligrams, is subjected to temperature changes, and highly sensitive temperature sensors record these temperature and energy variations. Phase transitions of materials, such as glass transition and melting, involve latent heat and changes in specific heat, requiring different amounts of energy during transitions. This results in varying electrical energy, which helps interpret physical transition temperatures.
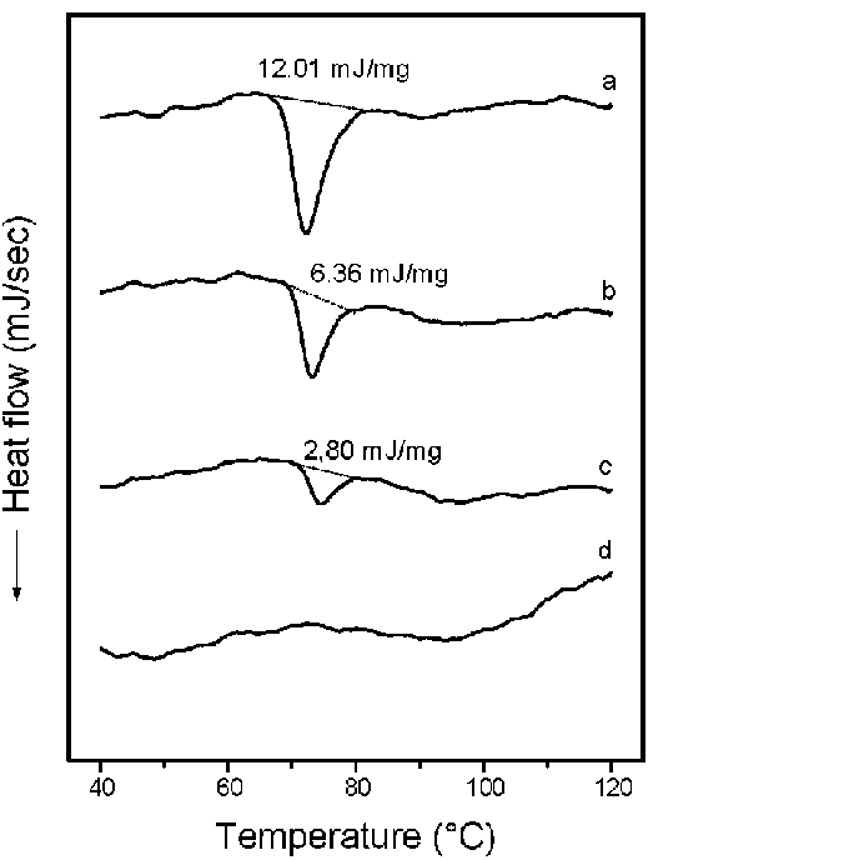
Starch and DSC
The thermal analysis of starch using DSC, involves preparing a starch suspension containing at least 80% water in a special sample container (sample pan). The sample container must be of the high-pressure type, sealed tightly. The sample is heated with a specific temperature gradient, compared to a reference sample that is empty. The energy flux-temperature graph is then plotted, showing transition points as peaks. If there is insufficient water in the sample environment, the melting patterns will differ. Thus, to obtain accurate and symmetrical results that represent starch melting behavior in most food products subjected to heat processing, an adequate amount of water is required.
Characteristics of Starch Crystallite Melting
- The melting process of amylopectin crystals is gradual and occurs at temperatures below the boiling point of water due to the different crystalline forms. Each observed peak consists of three index temperature phases: onset, peak, and end of melting.
- A similar pattern for melting amylopectin crystals is observed in starches containing amylose and fat molecules, such as corn starch. This peak generally appears at temperatures above 100°C.
- Another important parameter in the DSC curve for starch suspension is the energy required for melting or enthalpy of melting, determined by measuring the peak area and presented relative to the sample mass. The more extensive and perfection the crystalline structure of the starch, the higher the enthalpy of melting per unit mass.
- Retrogradation of starch leads to structural differences in the formed crystals and a reduction in their quantity compared to the initial values. Measuring the enthalpy of fusion in retrograded starch and comparing it to the initial values can serve as an indicator of the retrogradation or reformation of the crystalline structure.
- DSC can be used to analyze the intensity of amylose double-helix presence, which melts in the temperature range of 120°C to 200°C.
- This technique is also useful for analyzing staling or retrogradation in products like bread and cakes, where starch plays a key role.
For more information, refer to articles on the potential applications of high-amylose starch and instrumental techniques in formulation development.